・How do neurons generate information to drive behavior?
The Tabuchi lab's overarching goal is to elucidate how neural coding impacts molecular/cellular signaling, plasticity, and behavior.
・We employ multidisciplinary approaches, leveraging both Drosophila models and human iPS cell-derived neurons.
Through our research in Drosophila, we can precisely and rapidly identify significant phenotypes, and then validate the conservation of these phenotypes in human iPS cell-derived neurons.
The phenotype identification process involves the use of state-of-the-art data acquisition methods, including patch-clamp electrophysiology, multi-electrode array and FRET functional imaging, quantitative behavioral measurements, as well as our sophisticated machine learning platforms that aid in the construction of mathematical models to extract features of the temporal structure of neural activity patterns.
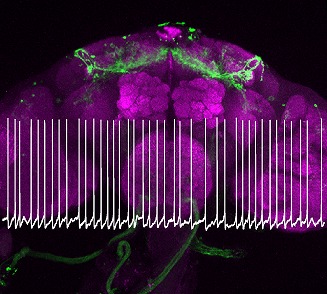
・Our research focuses on understanding the relationship between sleep quality and neural coding.
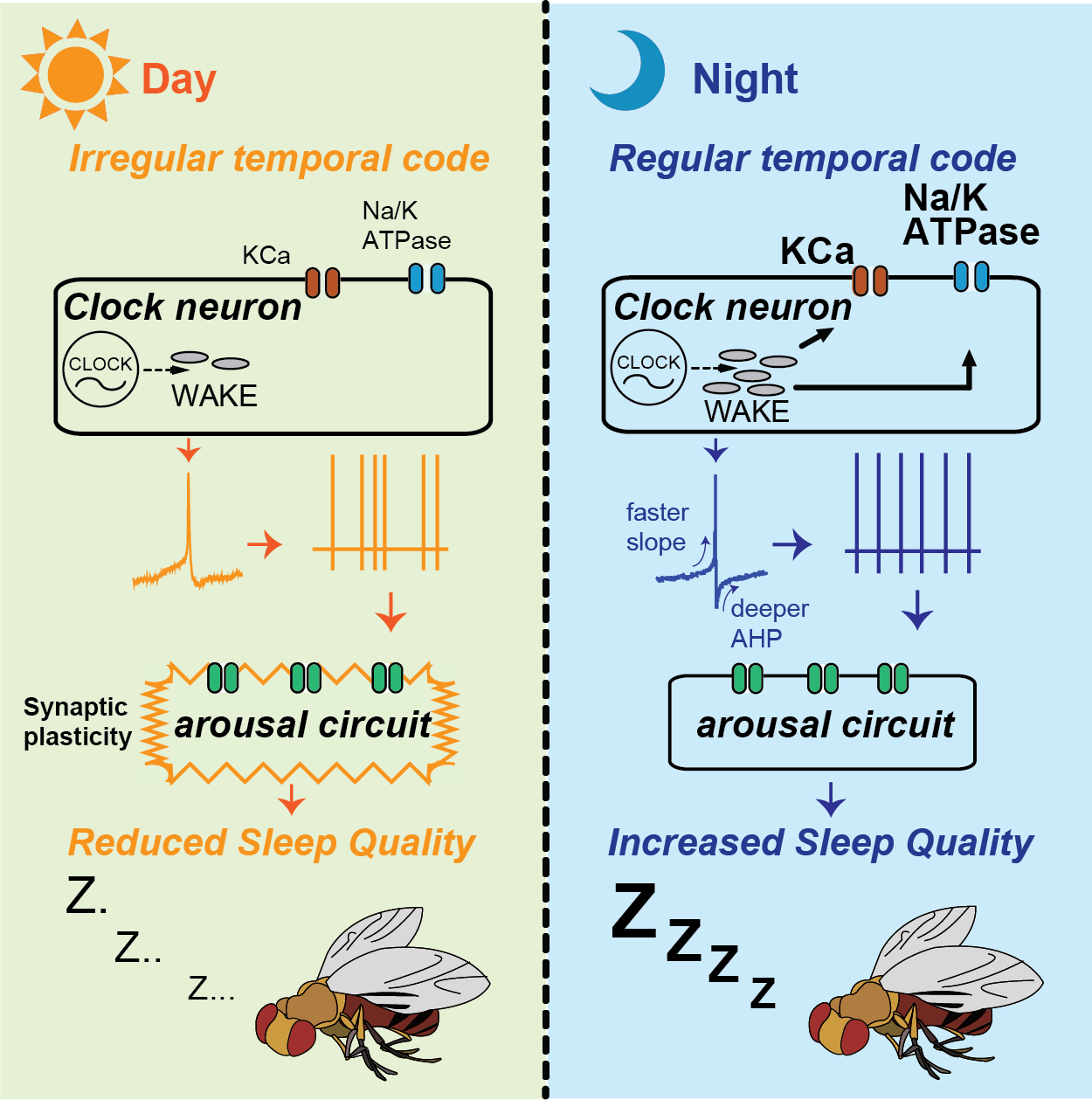
Sleep quality is influenced by clock-driven temporal coding within arousal circuits (Tabuchi et al., 2018), and previous work in our lab has revealed age-related unstructured patterns of this temporal coding (Nguyen et al., 2022).
We have also elucidated the molecular and biophysical mechanisms underlying the impact of downstream arousal circuits on temporal codes, identifying a novel form of synaptic plasticity driven solely by the temporal pattern of spiking.
Our current investigations aim to explore how neural codes affect molecular/cellular signaling to regulate sleep quality, employing techniques such as in vivo intracellular and extracellular electrophysiology, in vivo functional imaging, optogenetics, and sophisticated behavioral analysis.
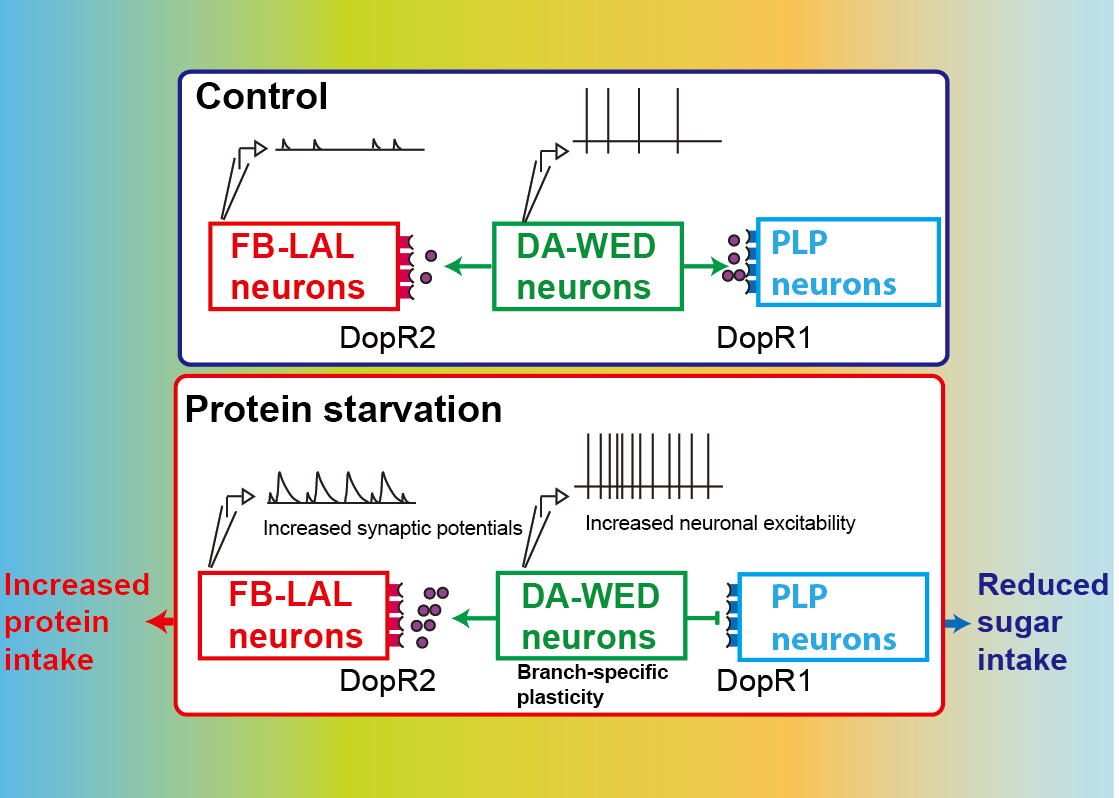
・Using a specific neural circuit that encodes protein-specific hunger (called DA-WED neurons) in Drosophila, we seek to understand how the temporal structure of membrane potential serves as a non-canonical (temporal or analog) neural code for the persistent internal drive of nutrition-specific hunger/satiety through a combination of molecular, genetic, computational, and electrophysiological approaches.
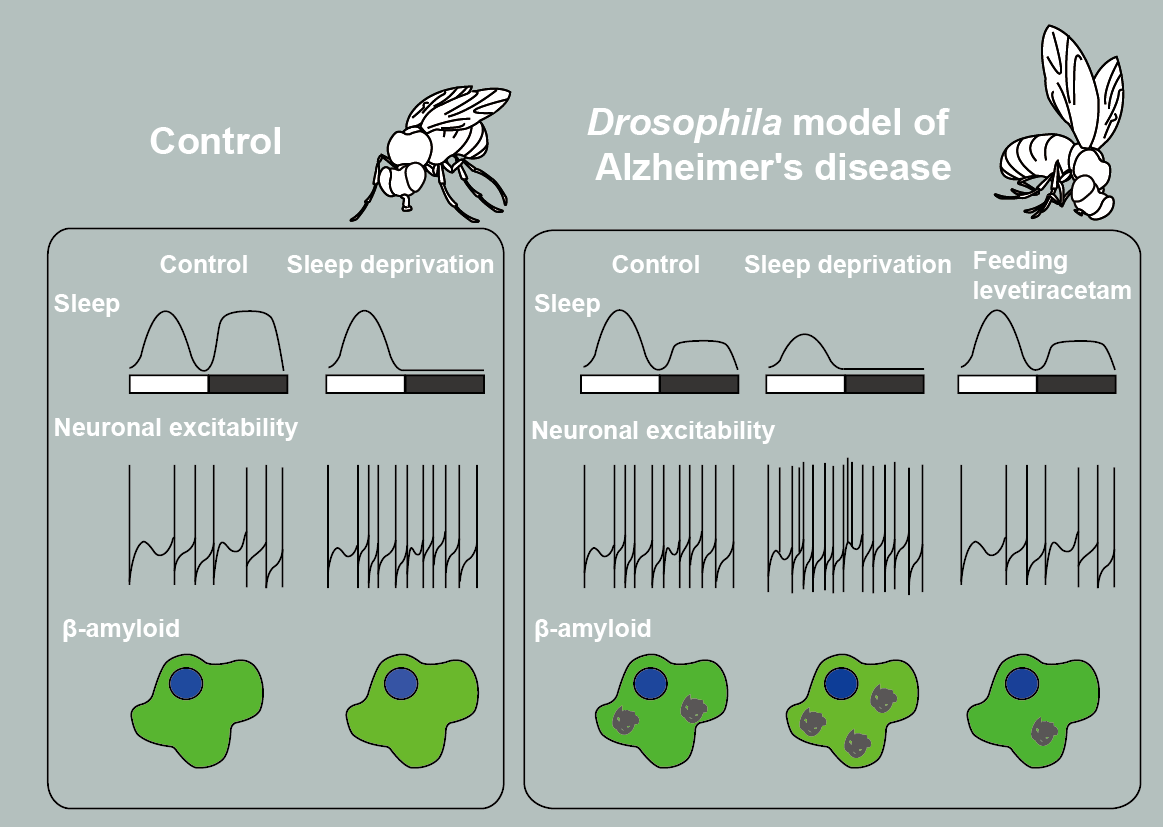
・We aim to uncover novel pathological mechanisms of Alzheimer's disease, Epilepsy, and Parkinson's Disease by integrating research in both Drosophila and human iPS cell-derived neurons.
Leveraging Drosophila models allows us to rapidly capture behaviorally relevant molecular phenotypes related to diseases, which we then validate in human iPS cell-derived neurons.
Our advanced data acquisition methodologies, including simultaneous electrophysiology and quantitative measurements, coupled with sophisticated machine learning platforms, enable us to construct mathematical models extracting features of the temporal structure of neural activity patterns.
Our study will address gaps in the understanding of how changes in computational biophysical states contribute to disease-causing factors and pave the way for the development of novel therapeutic strategies.
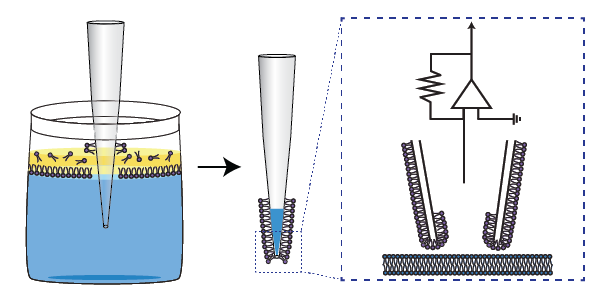
・We are interested in developing novel electrophysiological methods to facilitate long-term stable measurements of neuronal activity.
Electrophysiological recording with glass electrodes remains one of the most effective techniques for measuring membrane potential dynamics and ionic currents of voltage-gated channels in neurons.
We have pioneered the development of a phospholipid membrane coating on glass electrodes to improve the quality of intracellular electrophysiological recordings (Jameson et al., 2024), and we are exploring ways to extend this technology to various other electrode types.
・Our research scope encompasses hierarchical interactions of gene expression, biophysical properties such as ionic currents, temporal structure of membrane potential dynamics including spiking patterns, functional synaptic connectivity of neural circuits, and behavior.
We aim to answer fundamental questions such as the determining factors of processing performance in neural circuits, the molecular and cellular events underlying these factors, and their modulation of neuronal, synaptic, and behavioral states.
By addressing these questions, we anticipate gaining key insights into the principles of neural codes underlying the persistent internal drive of the brain and providing a framework to understand how these factors contribute to overall health.
Thanks to the generous sponsors of our research





